Introduction: The Accidental Revolution
When Alexander Fleming returned to his London lab in September 1928, he found a contaminated Petri dish with a mysterious mold (Penicillium notatum) dissolving deadly Staphylococcus colonies. This serendipitous discovery birthed the antibiotic era, saving an estimated 200 million lives to date. Yet today, misconceptions about allergies and antibiotic resistance threaten its legacy. This guide bridges scientific accuracy with clinical insights you can trust.
1. Mechanism of Action: Precision Targeting
The Beta-Lactam Breakthrough
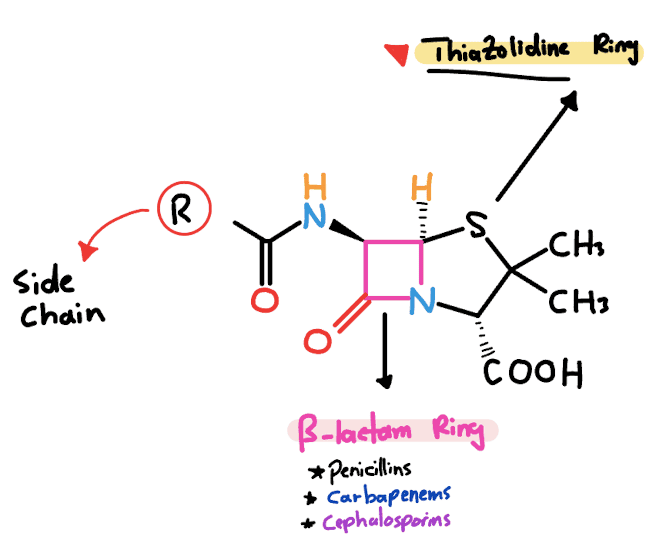
Penicillin’s core structure features a β-lactam ring – a strained four-atom ring that enables its bactericidal activity.
Step-by-Step Process:
- Binding: Penicillin binds to penicillin-binding proteins (PBPs) on bacterial cell walls.
- Inhibition: It blocks transpeptidase enzymes responsible for cross-linking peptidoglycan chains.
- Structural Collapse: Uncross-linked cell walls weaken → osmotic pressure ruptures bacteria.
Safety in Humans: Mammalian cells lack peptidoglycan, ensuring selective toxicity.
Resistance Evolution: Three Molecular Pathways
- β-lactamase Enzymes:
- Mechanism: Hydrolyze the β-lactam ring (e.g., TEM-1 enzyme in E. coli).
- Countermeasure: Clavulanic acid or tazobactam inhibit these enzymes.
- Modified PBPs:
- Example: PBP2a in MRSA reduces drug affinity 1000-fold.
- Porin Channel Alterations:
- Impact: Gram-negative bacteria limit drug entry (e.g., Pseudomonas aeruginosa).
2. Penicillin Classifications: Beyond the Basics
Structural Adaptations Define Clinical Utility
| Class | Prototype Drugs | Spectrum & Key Uses | Pharmacokinetics |
|---|---|---|---|
| Natural Penicillins | Penicillin G, V | Streptococci, syphilis, meningitis | G: IV/IM; V: Oral (acid-stable) |
| Penicillinase-Resistant | Nafcillin, Dicloxacillin | MSSA skin/soft tissue infections | Oral/IV; 90% protein-bound |
| Aminopenicillins | Amoxicillin, Ampicillin | H. influenzae, UTIs, otitis media | Amoxicillin: 95% oral bioavailability |
| Antipseudomonal | Piperacillin-tazobactam | Pseudomonas, ICU sepsis | IV only; renal dose adjustment |
Clinical Pearls:
- Penicillin V: First-line for streptococcal pharyngitis (prevents rheumatic fever).
- Piperacillin-tazobactam: Empiric therapy for hospital-acquired pneumonia (95% gram-negative coverage).
3. Adverse Effects: Evidence-Based Management
Hypersensitivity Reactions: A Tiered Approach
Penicillin Hypersensitivity Reactions: From Rashes to Life-Threatening Emergencies
1. Immediate (Type I) IgE-Mediated Reactions
Onset: Within minutes to 1 hour after exposure.
Key Features:
Anaphylaxis: Sudden hypotension, bronchospasm, or stridor.
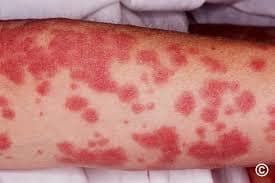
Urticaria/Angioedema: Raised, itchy welts without fever.
Management:
- Epinephrine (0.3–0.5 mg IM in thigh) first-line.
- ICU monitoring for 24 hours (risk of biphasic reactions).
2. Delayed (Type IV) T-Cell Reactions
Onset: 4–14 days after starting therapy.
Key Features:
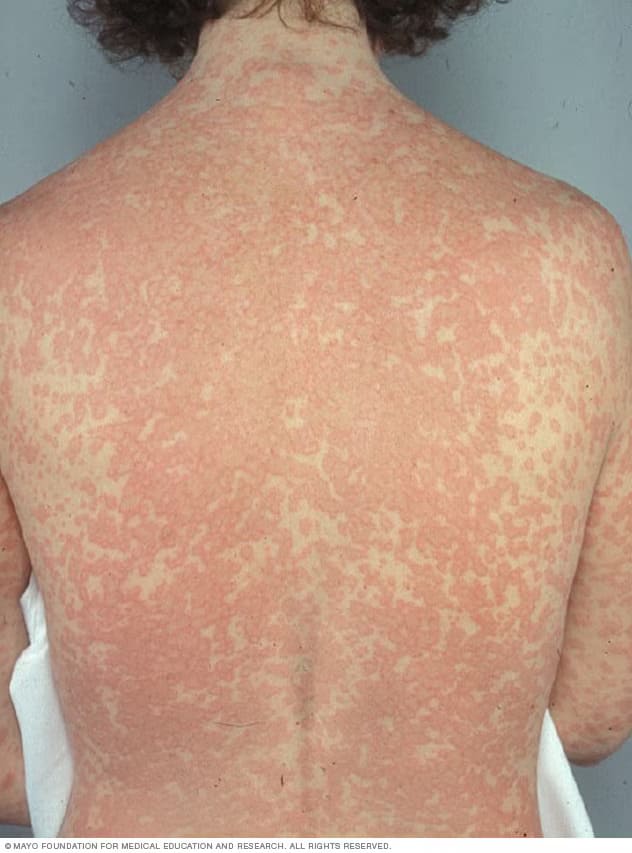
- Maculopapular Rash: Flat, red, non-blanching spots (5–10% with amoxicillin).
- No Systemic Symptoms: Differentiates from SJS/TEN.
Management: - Discontinue penicillin; oral antihistamines (e.g., loratadine) for pruritus.
- Critical Clarification: Not a true allergy—rechallenge possible if needed.
SEO Note: Targets “amoxicillin rash vs allergy,” “can I take penicillin after a rash.”
3. Severe Cutaneous Reactions: SJS & TEN
Onset: 1–3 weeks (often after 7–10 days of therapy).
Steven-Johnson Syndrome (SJS):
- Early Signs: Fever, sore throat, conjunctival redness (mimics flu).
- Rash Evolution: Painful targetoid lesions → skin sloughing (<10% BSA).
- Pathognomonic Sign: Nikolsky sign (epidermal detachment with lateral pressure).
Toxic Epidermal Necrolysis (TEN) 🚨:
- Progression: Similar to SJS but with >30% BSA detachment.
- Mortality: 30–40% (vs. 10% for SJS).
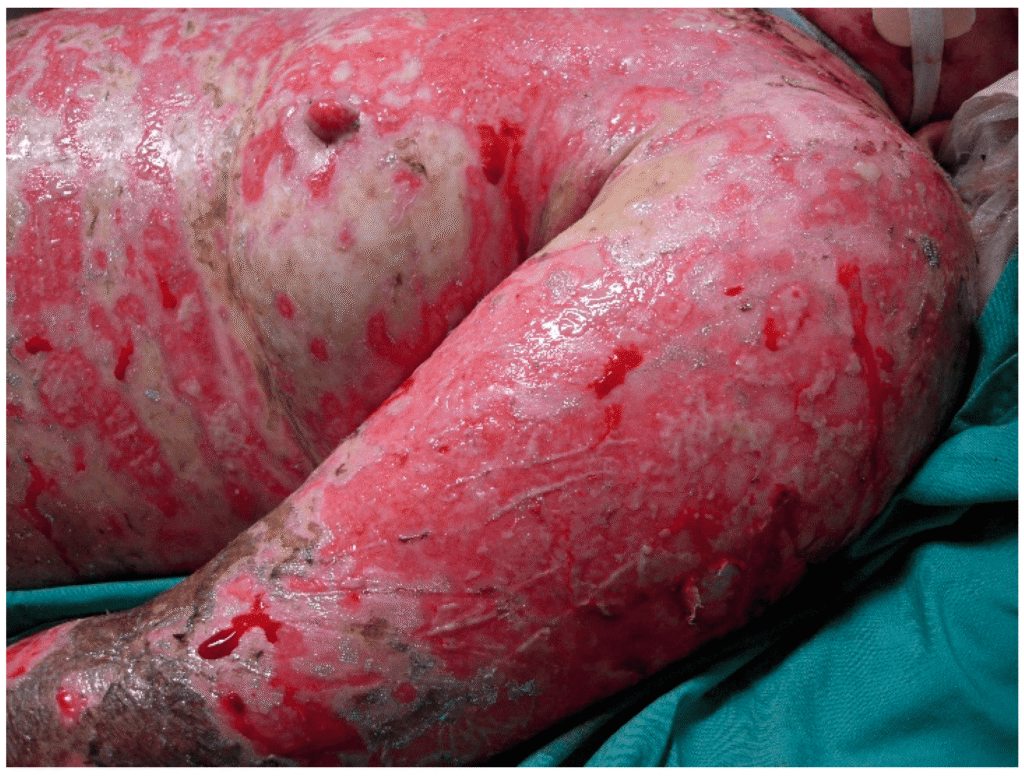
Toxic Epidermal Necrolysis (TEN)
Management:
- Immediate Actions:
- Hospitalize in burn unit (fluid/electrolyte support).
- IVIG 1–2 g/kg/day or cyclosporine (controversial but used).
- Avoid: Corticosteroids (increased infection risk).
- Diagnostic Tool: SCORTEN scale (predicts mortality based on age, BSA, lab values).
SEO Note: Targets “SJS vs TEN penicillin,” “Nikolsky sign photo,” “IVIG protocol for SJS.”
Systemic Complications
- Interstitial Nephritis:
- Diagnostic Triad: Fever + rash + eosinophilia (urine/serum).
- Treatment: Drug cessation ± corticosteroids.
- C. difficile Colitis:
- Risk Reduction: Avoid unnecessary broad-spectrum use; probiotics.
Critical Data: 90% of “penicillin allergies” are misdiagnosed. Skin testing reduces alternative antibiotic use by 50% (JAMA Intern Med 2023).
4. Resistance Crisis: Data-Driven Solutions
Global Threat Landscape
- 2.8 million antibiotic-resistant infections/year in the US (CDC).
- 35,000 deaths annually from resistant pathogens.
- Top Threats: MRSA, carbapenem-resistant Enterobacteriaceae, Pseudomonas.
Preservation Strategies
- Antibiotic Stewardship:
- Use narrow-spectrum agents (e.g., penicillin for strep throat).
- Adhere to 5-day courses for uncomplicated infections.
- Diagnostic Innovations:
- Rapid MALDI-TOF for bacterial ID (<1 hour).
- Procalcitonin-guided therapy discontinuation.
- Novel Combinations:
- Meropenem-vaborbactam for KPC-producing Klebsiella.
5. Special Populations: Tailored Therapy
Pregnancy & Pediatrics
- Pregnancy (Category B): Safest antibiotic class (no teratogenic risk).
- Children:
- Amoxicillin: First-line for AOM (80 mg/kg/day split BID).
- Avoid tetracyclines in <8 years.
Renal/Hepatic Impairment
- Renal Dosing:
- Penicillin G: Reduce frequency if CrCl <30 mL/min.
- Piperacillin: Extend infusion to 4 hours in dialysis.
- Hepatic: No adjustment needed (minimal liver metabolism).
6. Future Directions: Innovating Beyond Tradition
Pipeline Agents (2024 Clinical Trials)
- Cefepime-taniborbactam: Phase 3 for UTI/cUTI (against AmpC/ESBL producers).
- Sulbactam-durlobactam: Targeting carbapenem-resistant Acinetobacter.
Non-Traditional Approaches
- Antibiotic Adjuvants:
- Avibactam restores activity against OXA-48 carbapenemases.
- Bacteriophage-Penicillin Synergy:
- Phages disrupt biofilms → enhance penicillin penetration (Nature 2023).
Conclusion: Honoring Legacy Through Responsibility
Penicillin’s 95-year journey exemplifies science’s power—and its fragility. Preserving efficacy demands:
- Patient Education: Debunk allergy myths via skin testing programs.
- Provider Action: Adhere to IDSA stewardship guidelines.
- Policy Shifts: Support the PASTEUR Act for novel antibiotic development.
Final Quote:
“Without urgent action, we are heading toward a post-antibiotic era where common infections kill.”
— Dr. Tedros Adhanom Ghebreyesus, WHO Director-General
Check our video on YouTube: